Discussion
Discussion
Rituximab for ANCA-associated vasculitis
Last updated July 25, 2024, by Marisa Wexler, MS

What is rituximab for ANCA-associated vasculitis?
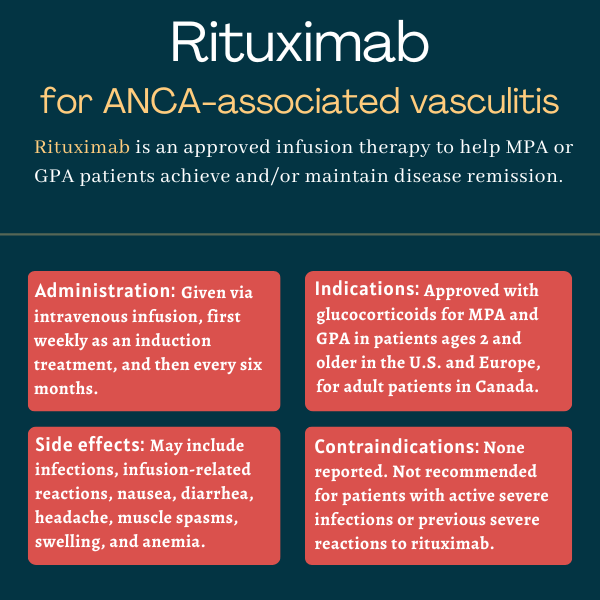
Rituximab is an infusion therapy approved for children and adults with granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA), the two most common types of ANCA-associated vasculitis (AAV).
The therapy, in combination with standard glucocorticoids, works to help induce and maintain disease remission in people with these autoimmune conditions.
Rituximab, originally discovered by Biogen, is sold under the brand name Rituxan in the U.S., Canada, and Japan, and as MabThera elsewhere. It is marketed by Biogen along with Genentech (a subsidiary of Roche) in the U.S., and by Roche in most other regions.
Several approved biosimilar versions of rituximab also are available in the U.S.; these medicines have been proven to be equivalent to the brand-name therapy in terms of quality, safety, and efficacy. Among them are Riabni, Ruxience, and Truxima. Much like generics, biosimilars are typically less expensive than the original medication.
Rituximab is widely approved for treating certain blood cancers and many other autoimmune diseases that share some underlying mechanisms, namely the involvement of certain cells, with AAV.
Therapy snapshot
| Brand name: | Rituxan |
| Chemical name: | Rituximab |
| Usage: | Used in combination with glucocorticoids to treat MPA and GPA patients; can help achieve and sustain disease remission |
| Administration: | Intravenous infusion |
How does rituximab work?
ANCA-associated vasculitis is an autoimmune disease marked by inflammation and damage to small blood vessels that can affect the function of several organs, most commonly the kidneys and lungs.
Most cases are associated with the production of self-reactive antibodies called antineutrophil cytoplasmic autoantibody, or ANCAs, that ultimately drive the erroneous inflammatory attack that causes damage to small blood vessels.
Rituximab is designed to destroy B-cells, the immune cells responsible for the production of antibodies — both those that normally help the body fight invaders and the abnormal antibodies that drive AAV and other autoimmune diseases.
The therapy itself contains an antibody that targets CD20, a protein highly present at the surface of B-cells. Its binding to CD20 on the surface of a B-cell triggers a cascade of molecular reactions that ultimately kills the cell.
By eliminating B-cells, rituximab is expected to reduce levels of ANCAs and dampen the inflammatory attack that drives MPA and GPA, ultimately easing their symptoms and reducing the risk of disease relapse.
Who can take rituximab?
Rituximab, in combination with glucocorticoids, was approved by the U.S. Food and Drug Administration in 2011 to treat adults with MPA and GPA. The therapy’s indication was expanded in September 2019 to also cover children with these conditions as young as age 2.
The treatment combination also is approved in Europe for inducing disease remission in children and adults with severe, active MPA or GPA, and in Canada to promote remission in certain adults with severely active MPA or GPA.
Who should not take rituximab?
The prescribing information for rituximab does not list any contraindications.
However, it carries a boxed warning noting it may increase the risk of serious and potentially life-threatening infusion-related reactions, infections, and reactions affecting the skin and the membranes lining some organs and body cavities.
Rituximab should generally not be used in patients who have previously experienced serious or life-threatening side effects while on the therapy. It further is not recommended for use in patients with severe, active infections.
How is rituximab administered in ANCA-associated vasculitis?
Rituximab is available as a colorless, clear solution in single-dose vials of 10 or 50 mL, each containing 10 mg rituximab per mL of liquid. It is administered via infusion into the bloodstream by a healthcare professional, with appropriate support on hand to manage any infusion-related reactions that may occur.
Treatment with rituximab for MPA and GPA involves an initial induction phase that aims to bring the disease under control. If successful, that is followed by maintenance treatment designed to keep the disease in remission.
Induction treatment in both adults and children with active disease involves an infusion, at a dose of 375 mg per square meter (mg/m2), given once weekly over four weeks.
Within two weeks before starting rituximab, and no later than the first infusion, patients should also receive glucocorticoids, a type of anti-inflammatory and immunosuppressive medication commonly used to help induce remission in AAV.
This includes three days of intravenous methylprednisolone — at a daily dose of 1,000 mg for adults and 30 mg/kg (not exceeding 1 g/day) for children — followed by oral prednisone, or its equivalent, per clinical practice.
Maintenance treatment should be started 16 to 24 weeks (about 3.5 to 5.5 months) after the last induction infusion. It involves two infusions given two weeks apart, then additional infusions every six months as needed. The dose for each maintenance infusion is 500 mg for adults and 250 mg/m2 for children.
Patients may sometimes receive other medications as an induction treatment to control the disease, followed by maintenance treatment with rituximab for long-term control. In these cases, rituximab maintenance treatment should follow the same schedule and should be started within four weeks after disease control is achieved with the other medications.
Rituximab in clinical trials
Rituximab’s approval in the U.S. for adults with severe GPA and MPA was based largely on data from a Phase 2/3 clinical trial, called RAVE (NCT00104299) and sponsored by the National Institute of Allergy and Infectious Diseases.
RAVE
The RAVE study tested rituximab against standard treatment in 197 adults with either newly diagnosed or relapsing GPA or MPA at sites in the U.S. and the Netherlands. All of the patients were positive for ANCAs and 75% had GPA.
The participants were randomly assigned to undergo six months of induction treatment and a yearlong maintenance therapy period using either rituximab (375 mg/m2 once a week for four weeks) followed by a placebo, or cyclophosphamide followed by azathioprine.
Cyclophosphamide is a chemotherapy commonly used in AAV to kill B-cells, and azathioprine is an immunosuppressive treatment. Each patient also was treated with intravenous glucocorticoids followed by oral glucocorticoids that were gradually tapered off.
The study’s main goal was to see if the rituximab-based regimen was at least as good as standard treatment at bringing patients into remission. This was defined as a Birmingham Vasculitis Activity Score for Granulomatosis with Polyangiitis (BVAS/GPA) score of zero, indicating no disease symptoms, and successful glucocorticoid taper.
After the six-month induction period, the remission rate was 64% with rituximab and 53% with standard treatment, a nonsignificant difference, the results showed. This meant that rituximab was at least not inferior to standard care at inducing disease remission.
In addition, the number of patients still in remission through study months 12 and 18 also was comparable between groups, at 38% and 31%, respectively.
Among patients with relapsing disease, rituximab was associated with a significantly higher remission rate than standard therapy at both six months (67% vs. 42%) and 12 months (49% vs. 24%), but not 18 months (37% vs. 20%).
MAINRITSAN trial
A Phase 3 trial, dubbed MAINRITSAN (NCT00748644), tested rituximab as a maintenance therapy in newly diagnosed or relapsing AAV patients, 21 years and older, who had achieved remission with cyclophosphamide and glucocorticoids.
Funded by the French Ministry of Health, the study enrolled 115 patients at a single French site: 87 with GPA, 23 with MPA, and five with kidney-limited AAV. All were randomly assigned to receive maintenance therapy with either rituximab or azathioprine for up to 1.5 years. Rituximab was given at a fixed 500 mg dose, on the first day, two weeks later, and every six months thereafter.
The trial’s main goal was to assess the percentage of patients experiencing a major disease relapse, classified as either symptom reappearance or worsening and involvement of at least one major organ, a life-threatening complication, or both.
The results at more than two years of follow-up showed that a significantly lower proportion of rituximab-treated patients had experienced a major relapse compared with those on azathioprine (5% vs. 29%).
Longer-term data, after about five years of follow-up, continued to demonstrate rituximab’s superiority over azathioprine. Specifically, significantly more patients treated with rituximab remained free from major relapses (71.9% vs. 49.4%) and all relapses (57.9% vs. 37.2%), and were still alive (100% vs. 93%).
Trial in children
Regulatory approvals of rituximab for children with MPA or GPA were based on findings from a Roche-sponsored Phase 2a study called Pediatric Polyangiitis and Rituximab Study (PePRS; NCT01750697).
The trial enrolled 25 children, ages 6-17, with newly-diagnosed or relapsing MPA or GPA at about dozen sites in North America and Europe. Most were girls (80%), were positive for ANCAs (88%), and had a GPA diagnosis (76%).
All children underwent induction treatment with rituximab and glucocorticoids for six months. Rituximab was given at a dose of 375 mg/m2, once per week for four weeks. After that six-month induction phase, participants could receive subsequent doses of rituximab and/or other therapies to achieve remission or control relapses.
The main goals of the PePRS study were to assess the therapy’s safety and pharmacological properties, with efficacy measures such as the remission rate as exploratory endpoints. Remission was defined as a score of zero on the Pediatric Vasculitis Activity Score (PVAS) and successful glucocorticoid taper, or a PVAS score of zero on two consecutive assessments separated by at least one month, irrespective of glucocorticoid dose.
Data indicated that more than half of the children (56%) were in remission at the end of the induction treatment. More than two-thirds of all children (68%) received additional rituximab treatment, and 36% were given more than one additional immunosuppressive therapy. The remission rate was 92% at one year and 100% at 1.5 years.
The medication’s safety profile in these pediatric patients was similar to that seen in adult patients.
Ongoing studies
An observational study called BRAVO (NCT05716334), run by scientists at McGill University, in Canada, is collecting data from 201 adults with MPA or GPA who are treated with brand-name Rituxan or rituximab biosimilars to compare outcomes like relapse rates and safety measures. The study is slated to end in 2025.
The French Ministry of Health launched a Phase 3 trial, called MAINRITSEG (NCT03164473), to test rituximab as a maintenance therapy in adults with eosinophilic granulomatosis with polyangiitis (EGPA), the rarest form of AAV.
A total of 98 EGPA patients who achieved disease remission were recruited at a single French site. Participants were randomly assigned to receive two years of maintenance treatment with either rituximab (four infusions of 500 mg given six months apart) or the standard of care azathioprine.
The study’s main goal is to compare the amount of time patients are in remission with either treatment. That trial is expected to end in late 2024.
Common side effects of rituximab
The most common side effects of rituximab in people with MPA or GPA include:
- infusion-related reactions
- nausea
- diarrhea
- headache
- muscle spasms
- anemia
- swelling
- infections.
Infusion-related reactions
Rituximab carries a boxed warning noting that it may lead to potentially fatal reactions during or shortly after infusions of the medication. These severe to fatal infusion-related reactions usually develop 30 minutes to about two hours after the first infusion.
Symptoms can include hives, low blood pressure, swelling of the face, mouth, and/or tongue, severe allergic reaction, and breathing problems, as well as palpitations, and chest pain.
To reduce the risk of infusion reactions, patients should receive premedication with an antihistamine, acetaminophen, and a glucocorticoid before each infusion.
Patients with pre-existing lung or heart problems and those who have had previous heart- and/or lung-related adverse reactions should receive especially close monitoring for infusion-related reactions while on rituximab.
If an infusion-related reaction does occur, appropriate supportive care should be given at once. Depending on the reaction’s severity and the needed interventions, rituximab treatment should either be paused or discontinued permanently. If treatment is resumed after symptoms have resolved, the infusion rate should be slowed by at least 50%.
Skin and mucus membrane reactions
The therapy’s boxed warning also mentions the possible development of severe to fatal mucocutaneous reactions, or those affecting the skin and mucus membrane. Mucus membranes are those lining some body cavities and organs, such as the mouth, throat, lungs, and stomach.
Symptoms of mucocutaneous reactions that may occur due to rituximab include skin peeling, rash, blisters, pus-filled blister-like sores on the skin, and painful sores on the skin, lips, or mouth. The development of any such symptom should prompt patients to immediately inform their physicians or get medical help.
Rituximab should be discontinued if a severe mucocutaneous reaction develops while on the therapy.
Hepatitis B virus reactivation
Rituximab’s boxed warning also notes that, due to its immunosuppressive effects, the drug can trigger reactivation of the hepatitis B virus (HBV) — the cause of hepatitis B, a liver disease — even in those with an apparently resolved infection.
HBV reactivation, in which the virus grows much faster and can potentially cause life-threatening liver damage, has been reported up to two years after stopping rituximab treatment.
Patients should be screened for HBV infection before starting on rituximab. Those with current or prior infection should be monitored for reactivation during treatment and for several months after stopping. Antiviral therapy also may be given as per physician indication.
If HBV reactivation occurs, rituximab should be immediately stopped and appropriate supportive therapies should be given. The decision of whether to resume rituximab treatment in patients who have experienced HBV reactivation should be made in consultation with an expert in HBV.
Progressive multifocal leukoencephalopathy
Finally, the boxed warning also notes that rituximab may cause progressive multifocal leukoencephalopathy, or PML, a rare viral infection of the brain that can be fatal, especially in patients given prior or simultaneous immunosuppressive treatment. Such infections occurred more frequently within one year of the last rituximab dose.
Patients taking rituximab should be checked for PML if they start experiencing any unexplained neurological issues. If the condition is confirmed, the therapy should be discontinued.
Other infections, vaccination, blood counts
Because of its suppressive effects on the immune system, rituximab also can increase the risk of serious and fatal infections caused by other viruses, as well as bacteria and fungi. If a severe infection occurs, rituximab should be stopped and appropriate supportive care should be given. Patients with active severe infections should not receive treatment with rituximab.
Due to the infection risk, patients should not receive any vaccines containing a live virus before or during treatment with rituximab. Also, if possible, patients should be brought up to date on all non-live vaccines at least a month before starting on rituximab.
Moreover, all types of blood cells should be measured before the first rituximab infusion and regularly during treatment.
Heart problems
Rituximab may cause abnormal heart rhythms, known as arrhythmias, chest pain, and heart attack. Monitoring of heart function during and after rituximab infusions is required for patients with a history of heart issues or who have previously experienced clinically significant arrhythmias while on the therapy. If a serious arrhythmia develops, rituximab should be discontinued.
Combination with other medications
Rituximab for use in GPA and MPA has been rigorously studied in combination with glucocorticoids, but not with other immunosuppressive medications. If such combination treatments are given, patients should be closely monitored for any new potential adverse events.
In addition, abdominal pain, as well as bowel obstruction and perforation, have been reported in patients treated with rituximab and chemotherapy agents. Patients should report pain to their healthcare team.
Use in pregnancy and breastfeeding
Based on human data, the use of rituximab during pregnancy can cause issues for the developing fetus. This can include abnormally low B-cell counts, which can increase the risk of serious infections after birth. The risks and benefits of taking rituximab during pregnancy should be carefully weighed by patients and their healthcare teams prior to beginning treatment.
Patients who have the potential to become pregnant are advised to use an effective form of contraception while taking rituximab and for at least a year after stopping the therapy.
Babies who were exposed to rituximab during pregnancy should be closely monitored for signs of infection.
Rituximab has not been rigorously studied in people who are breastfeeding, but the therapy may be secreted in low levels into human breast milk. Given that it remains unknown whether this poses a risk for a breastfeeding infant, the FDA advises against breastfeeding while taking rituximab and for at least six months after the last dose.
Note: ANCA Vasculitis News is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
Recent Posts
- What a hip replacement taught me about bone health and vasculitis
- Cancer risk 23% higher in people with AAV, large US study finds
- Asthma drug dupilumab may ease symptoms in EGPA: Small study
- A valentine for the people who show up in our lives with ANCA vasculitis
- Immune cell surge tied to inflammation and disease activity in active GPA
Related articles
-
 Discussion
Discussion
-