Nucala (mepolizumab) for ANCA-associated vasculitis
Last updated May 31, 2024, by Marisa Wexler, MS

What is Nucala for ANCA-associated vasculitis?
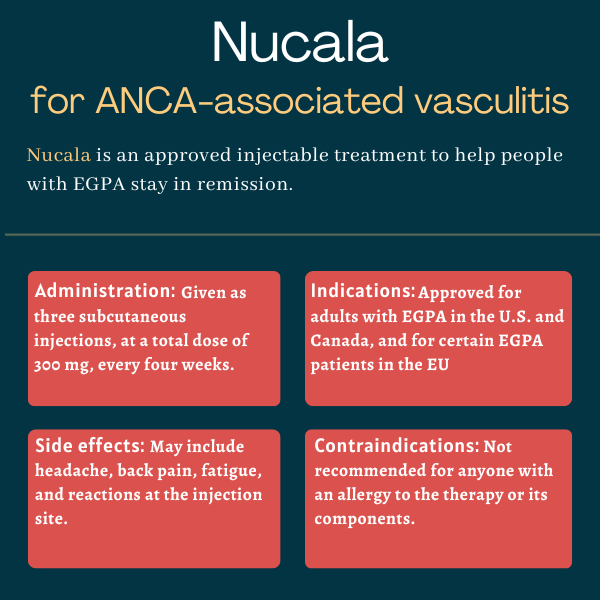
Nucala (mepolizumab) is an under-the-skin, or subcutaneous, injectable therapy approved for adults with eosinophilic granulomatosis with polyangiitis (EGPA), the rarest form of ANCA-associated vasculitis.
Sold by GSK (formerly GlaxoSmithKline), the therapy is used as an add-on to standard care with glucocorticoids. Nucala helps patients remain in remission, lowering their risk of relapses, or disease flares, and their need for glucocorticoids.
Nucala is also approved for conditions that share similar underlying immune-related mechanisms as EGPA, including some types of asthma and nasal inflammation, as well as a blood disorder called hypereosinophilic syndrome.
Therapy snapshot
| Brand name: | Nucala |
| Chemical name: | Mepolizumab |
| Usage: | Used to treat adults with EGPA; can help prolong disease remission |
| Administration: | Subcutaneous injection |
How does Nucala work?
ANCA-associated vasculitis, or AAV, is a group of autoimmune disorders characterized by inflammation in small blood vessels. That inflammation eventually damages tissues and organs in the body, and causes a wide array of disease symptoms.
EGPA is characterized by small clumps of inflammatory cells made up mostly of an immune cell called eosinophils that help drive the damaging inflammation in small blood vessels.
All EGPA patients have very high eosinophil levels at some point in their disease course, and nearly all have asthma. Besides the lungs, the gastrointestinal tract is also affected by the disease.
Nucala is an antibody-based therapy designed to block interleukin-5 (IL-5), a signaling molecule that promotes the growth, maturation, and survival of eosinophils. By reducing the levels and activity of eosinophils, Nucala is expected to lessen the inflammation that drives EGPA, ultimately prolonging disease remission and reducing the risk of relapse.
The therapy is also expected to reduce the need for glucocorticoids, a mainstay AAV treatment due to its anti-inflammatory and immunosuppressive effects, but whose high doses and long-term use are associated with serious side effects.
Who can take Nucala?
The U.S. Food and Drug Administration approved Nucala for adults with EGPA in December 2017, making it the first targeted medication to be cleared in the country for the rare autoimmune disease.
Nucala is also approved as an add-on treatment for adults with EGPA in Canada and for patients, 6 and older, with relapsing or refractory (treatment-resistant) EGPA in the European Union.
Who should not take Nucala?
Nucala should not be taken by anyone with a known allergy to the medication or any of its ingredients.
How is Nucala administered in ANCA-associated vasculitis?
For people with EGPA, Nucala is administered via subcutaneous injections, at a recommended dose of 300 mg, once every four weeks.
Nucala is available in 100 mg single-dose vials as a white to off-white powder, which must be reconstituted and injected by a healthcare professional.
It is also available as a single-dose prefilled autoinjector and a single-dose prefilled syringe, each containing 100 mg of the therapy in 1 mL of liquid that is clear to opalescent in sheen and colorless to pale yellow to pale brown in color. The prefilled autoinjector and syringe can be used by patients and caregivers after appropriate training by a healthcare provider.
Nucala’s recommended 300 mg dose is administered as three separate injections, each containing 100 mg of the therapy.
The injections may be done on the front of the thighs or the abdominal area (stomach) — but not within 2 inches (about 5 cm) of the belly button. The back of the upper arm can be used as an injection site if the medication is administered by a caregiver or a healthcare professional. Each injection should be given 2 inches apart from the other injection sites.
Before administration, the prefilled autoinjector or syringe should be removed from the refrigerator and allowed to warm at room temperature for about 30 minutes. No other warming methods should be used. If needed, Nucala can be stored at room temperature for up to seven days.
After removal from the carton, Nucala’s autoinjector or prefilled syringe must be administered in the next eight hours or discarded after that period.
The injection site — which should not be bruised, red, tender, or hard — should first be disinfected. The autoinjector should be held against the skin for up to 15 seconds after the start of the injection. The prefilled syringe’s needle should be inserted at a 45º angle into a pinched skin site, and the plunger should be slowly pushed all the way down to make sure all the medicine is injected.
If a planned dose of Nucala is missed, a new dose should be given as quickly as possible, and monthly dosing should resume on the usual day of administration. If the next dose is already due, the therapy should be administered as planned.
Nucala in clinical trials
Nucala’s approvals were based mainly on data from an international Phase 3 clinical trial called MIRRA (NCT02020889).
MIRRA
The GSK-sponsored MIRRA study evaluated Nucala’s safety and effectiveness against a placebo in 136 adults with relapsing or refractory EGPA.
Relapsing disease was defined as a history of at least one relapse within the past two years while on glucocorticoid treatment. Refractory EGPA was defined as either failure to achieve disease remission within the past six months following standard induction treatment, given for three months or longer; or EGPA symptom recurrence in the past six months, while tapering off glucocorticoids.
Participants were randomly assigned to receive subcutaneous injections of either Nucala (300 mg) or a placebo, once every four weeks for 52 weeks (one year). All were allowed to continue their standard treatments, including oral glucocorticoids such as prednisolone and prednisone, with or without immunosuppressive therapy. The dosage of glucocorticoids could be tapered off at the discretion of trial investigators.
MIRRA’s main goals were to assess the total accumulated time that participants were in remission and the proportion of patients in remission at weeks 36 and 48. Disease remission was defined as a score of zero on the Birmingham Vasculitis Activity Score, a standard measure of AAV activity, while taking prednisolone or prednisone at minimal doses (up to 4 mg/day).
Results showed 81% of patients on the placebo and 47% of those on Nucala did not achieve remission at all.
Those given Nucala were in remission for a significantly longer accumulated time than those on the placebo. In particular, a significantly greater proportion of Nucala-treated patients were in remission for at least 24 weeks (six months) relative to those given the placebo (28% vs. 3%). This difference indicated a nearly six times higher likelihood of achieving remission with Nucala than with a placebo.
In addition, nearly one in three (32%) of the patients on Nucala were in remission at weeks 36 and 48, compared with 3% of those on the placebo — a statistically significant difference.
Significantly more Nucala-treated patients achieved remission within the first 24 weeks and remained in remission until the end of treatment compared with those on the placebo (19% vs. 1%), reflecting a 19 times higher chance of maintaining remission with Nucala.
Moreover, the therapy was associated with a significantly lower chance, by 68%, of experiencing a disease relapse, and a 50% lower annualized relapse rate relative to the placebo.
Data also showed the overall patient population was receiving a median of 12 mg of oral glucocorticoids per day at the start of the study. Over the one-year period, those treated with Nucala were on a significantly lower daily dose of oral glucocorticoids, compared with those on the placebo (mean of 9.2 vs. 13.5 mg).
During the last month of treatment, most patients in the Nucala group (59%) were on a glucocorticoid dose of up to 7.5 mg per day, whereas two-thirds of those on the placebo (66%) were receiving a daily dose higher than 7.5 mg. A significantly greater proportion of patients treated with Nucala were able to discontinue glucocorticoids completely (18% vs. 3%).
Ongoing studies
A Phase 3 trial called E-merge (NCT05030155) is testing a Nucala-based treatment regimen against conventional immunosuppressive treatment — such as cyclophosphamide followed by azathioprine — to induce remission in up to 100 adults with active EGPA.
The study, being conducted at a single center in Paris, France, will involve newly diagnosed or relapsing EGPA patients. Participants will be randomly assigned to one of the treatment regimens, in addition to receiving a standardized glucocorticoid tapering schedule.
E-merge’s main goal is to assess the percentage of patients receiving a daily prednisone dose of up to 4 mg after 5.5 months of treatment, without experiencing a disease relapse. Secondary goals include additional efficacy measures associated with prednisone dosage, relapses, and disease activity, as well as safety measures, for up to one year of treatment.
The study is set to end in 2025.
Common side effects of Nucala
The most common side effects of Nucala in people with EGPA include:
- headache
- injection site reactions such as pain, redness, and/or swelling
- back pain
- fatigue.
Allergic reactions
Nucala may cause allergic reactions that can be serious and life-threatening. Symptoms may include swelling of the face, mouth, and/or tongue; breathing problems; low blood pressure that can lead to dizziness or lightheadedness, and fainting; rash; and hives.
Allergic reactions usually happen within hours of a Nucala injection, but there have been reports of reactions that don’t become apparent until a few days after dosing. If an allergic reaction occurs, treatment with Nucala should be immediately and permanently discontinued.
Viral and parasitic infections
People on Nucala may be at a higher risk of infections, given that the treatment reduces the levels and activity of eosinophils, which are involved in fighting bacteria, viruses, parasites (including helminths or worms), and other potential invaders.
Infections of herpes zoster, the virus that causes shingles and chickenpox and that causes infections more often in people with a weakened immune system, have been reported in Nucala-treated patients within clinical trials. Patients who are starting or already on the therapy should be vaccinated against this virus as appropriate.
Patients with active parasitic infections were excluded from Nucala trials, so it’s not known how the therapy affects these patients’ immune response to parasites, but there’s a theoretical reason to think it may worsen such infections.
Anyone with pre-existing helminth infections should receive appropriate treatment for the parasite before starting on Nucala. If patients on Nucala develop a helminth infection and do not respond to appropriate treatment, Nucala should be withheld until the parasitic infection resolves.
Considerations for reducing glucocorticoids
Although Nucala has been shown to lessen the need for glucocorticoids, abruptly stopping these other medications can lead to a dangerous withdrawal effect or reveal health conditions that were being controlled by glucocorticoids. As such, if glucocorticoid use needs to be reduced, they should be gradually tapered off under the supervision of a physician.
Acute asthma symptoms or exacerbations
Nucala should not be used to manage acute asthma symptoms or acute exacerbations of asthma. If asthma remains uncontrolled or if it worsens after starting on Nucala, patients should seek medical advice.
Use in pregnancy and breastfeeding
Nucala has not been rigorously studied in people who are pregnant or breastfeeding. Studies in monkeys showed no signs of harm to the fetus when Nucala was given during pregnancy at doses up to nine times the maximum recommended dose for people. Data from mice also have not shown the drug to cause harm when given during pregnancy.
It is also not known if Nucala can be found in human breast milk or whether it affects nursing infants or breastmilk production. However, data from animal experiments suggest the drug is present in breast milk.
Patients are advised to discuss with their healthcare team the potential benefits and risks of Nucala in these situations.
Note: ANCA Vasculitis News is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
Recent Posts
- Tapeworm medication may ease ear, nose, throat symptoms in AAV
- What a hip replacement taught me about bone health and vasculitis
- Cancer risk 23% higher in people with AAV, large US study finds
- Asthma drug dupilumab may ease symptoms in EGPA: Small study
- A valentine for the people who show up in our lives with ANCA vasculitis