Discussion
Discussion
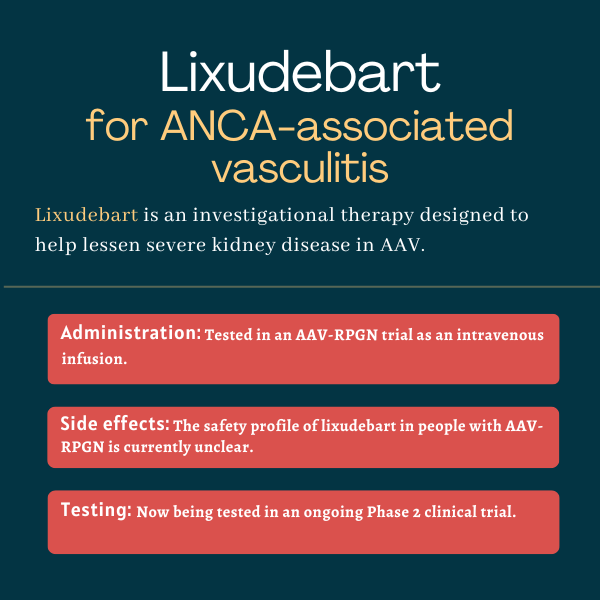
Lixudebart for ANCA-associated vasculitis
Last updated Feb. 26, 2025, by Marisa Wexler, MS

What is lixudebart for ANCA-associated vasculitis?
Lixudebart is a treatment candidate in the pipeline at Alentis Therapeutics that aims to help control serious kidney disease that arises as a complication of ANCA-associated vasculitis (AAV).
Given via intravenous, or into-the-vein, infusions, the therapy is expected to be beneficial for this subgroup of AAV patients by lessening kidney scarring, or fibrosis.
Alentis is also developing lixudebart as a potential treatment for other conditions marked by organ scarring, including advanced liver fibrosis and idiopathic pulmonary fibrosis, which is characterized by lung scarring.
Therapy snapshot
| Treatment name: | Lixudebart |
| Administration: | Being tested in AAV as an intravenous infusion |
| Clinical testing: | Currently in a Phase 2 clinical trial |
How does lixudebart work in ANCA-associated vasculitis?
AAV is a group of autoimmune disorders marked by inflammation in small blood vessels, which sets the stage for organ damage that drives disease symptoms. The kidneys, which house many small vessels, are often one of the organs most affected by AAV.
The most severe kidney manifestation of AAV is rapidly progressive glomerulonephritis, or RPGN, a condition marked by progressive inflammation and damage to the waste filtering units of the kidneys, called glomeruli. This can be accompanied by the buildup of scar tissue that further compromises kidney function and which can lead to kidney failure.
Lixudebart, formerly called ALE.F02, is an antibody-based therapy designed to prevent and reverse fibrosis by targeting a protein called Claudin-1, or CLDN1. Under normal conditions, Claudin-1 is hidden within tight junctions that allow cells to stick together. But when cells are damaged, CLDN1 gets exposed outside those tight junctions, triggering molecular signaling pathways that drive tissue scarring.
This system can theoretically help the body to form scar tissue to heal injuries, but in inflammatory diseases like AAV, continual inflammatory damage results in frequent Claudin-1 exposure, ultimately driving excessive buildup of extracellular matrix (ECM) components such as collagen.
ECM components surround cells and give structure to tissues. However, when overproduced, they lead to fibrosis. Increasing accumulation of collagen creates a physical barrier that can, over time, cause organ failure.
Lixudebart specifically binds to exposed Claudin-1 without interfering with the protein in tight junctions. By suppressing fibrotic signaling and opening the collagen barrier, the therapy is expected to lessen kidney damage in people with AAV-related RPGN, called AAV-RPGN.
How will lixudebart be administered in ANCA-associated vasculitis?
In the single trial to date testing lixudebart in AAV-RPGN patients, the therapy has been given through intravenous infusions at three different doses. Treatment involves 13 infusions given over the course of about six months.
Lixudebart in ANCA-associated vasculitis clinical trials
A Phase 2 clinical trial called RENAL-F02 (NCT06047171) is evaluating lixudebart’s safety, tolerability, and pharmacological properties against a placebo in an estimated 80 adults with recently diagnosed or suspected AAV-RPGN. The trial also is assessing the kidney effects of lixudebart.
The first participant was dosed in late 2023, with the study being conducted at sites in several European countries, as well as Turkey.
Participants overall were divided into four groups. Under the trial plan, the first group was being treated with a low dose of lixudebart, while the second received a high dose of the medication. The third group was receiving the maximum tolerable dose of lixudebart, as determined by results from the first two groups. The fourth group is being given a placebo infusion and will be used as a control group.
All patients will receive 13 infusions of their designated treatment for about six months, in addition to standard of care therapies for AAV-related kidney inflammation.
The study’s main goal is to assess lixudebart’s safety and tolerability, while a key secondary goal is any change in the mean estimated glomerular filtration rate (eGFR), a standard measure of kidney function. Changes in other measures of kidney function, including the presence of excessive protein in urine, known as proteinuria, are also being investigated.
Alentis announced interim results from the trial in early 2025. These concerned the first 26 participants who had been treated for up to six months. According to the company, lixudebart showed a favorable safety profile in these patients, and appeared to be associated with eGFR improvements and reductions in proteinuria, suggesting better kidney function. In agreement, data also showed reductions in the levels of CD163, a urinary biomarker of disease activity in AAV-glomeruloneprititis.
Common side effects of lixudebart
Given that full data from the Phase 2 RENAL-F02 trial have not yet been published, the safety profile of lixudebart in people with AAV-RPGN remains unclear.
ANCA Vasculitis News is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
Recent Posts
- What a hip replacement taught me about bone health and vasculitis
- Cancer risk 23% higher in people with AAV, large US study finds
- Asthma drug dupilumab may ease symptoms in EGPA: Small study
- A valentine for the people who show up in our lives with ANCA vasculitis
- Immune cell surge tied to inflammation and disease activity in active GPA
Related content
-
 Discussion
Discussion
-